FAQs
General Frequently Asked Questions
*These statements have not been evaluated by the FDA. Innate Healthcare Institute is a private clinic and any treatments utilizing any form of regenerative medicine is considered experimental.
What are stem cells and exosomes?
Mesenchymal Stem Cells (MSCs), commonly referred to just as stem cells, are multipotent cells that have the ability to become more than one cell type. While they have this ability to change into other cell types, it’s their ability to secrete bioactive molecules that make them so amazing at healing our bodies. MSCs release proteins called growth factors, trophic factors, cytokines, chemokines, and exosomes.
MSCs work by:
- Controlling inflammation
- Modulating the immune system
- Stimulating regeneration
- Reduce scarring
Exosomes are nanoparticle proteins secreted from stem cells (many cells secrete exosomes) in order to facilitate the healing capabilities of the surrounding cells. Exosomes induce progenitor stem cells to differentiate, so when injected locally or given intravenously they will deliver genetic material that tell surrounding cells to regenerate.
So simply put stem cells are a type of cell that produce medicine to heal other damaged cells and keep the environment around them balanced. They manage the bodies innate regenerative potential.
According to Arnold Caplan, PhD, considered to be the father of stem cell therapy since he discovered and named them Mesenchymal Stem Cells (MSCs). MSCs should more appropriately be named “Medicinal Signaling Cells” due to their most important function being the totality of secreted bioactive molecules.
“MSCs are multifactorial site-specific sensors with genetically wired molecular responses. MSCs see a signal and they respond in a controlled way. The MSC story will change the way medicine is practiced. Management of the patient’s innate regenerative resources will be the new treatment.” – Arnold Caplan
What kind of regenerative medicine products does Innate Healthcare use?
Stem cells and exosomes:
We use umbilical cord derived mesenchymal stem cells (UC-MSCs) from umbilical cord tissue (sometimes referred to as Wharton’s Jelly (WJ)). the gelatinous layer found within the umbilical cord, which is obtained from healthy, consenting donors who are under the age of 28 and undergoing a scheduled c-section. The FDA strictly regulates the process of how these tissues are acquired, tested, processed and stored to ensure the highest level of patient safety. The exosomes are derived from MSCs from the WJ. All processing is done in a clinical-grade laboratory by highly trained professionals.
All recovery, processing, labeling and distribution is in accordance with Good Manufacturing Practices (cGMP) and Good Tissue Practices (cGTP), established by the US Food & Drug Administration (FDA). The product is prepared aseptically and supplied sterile. We have never had an incident of contamination or severe adverse events.
Our stem cells are for the intended use of licensed physicians and exculsive to patients of Innate Healthcare Institute, and are not for sale or to be distributed commercially.
The UC-MSCs come from donated umbilical cords that meet eligibility determined for for donors of human cells, tissues, and cellular and tissue-based products, as defined in US FDA requirements for Human Cellular and Tissue based Products (HCT/P), 21 CFR 1271, State regulations and guidelines of the American Association of Tissue Banks (AATB) and AABB. This medicine is used at the discretion of a licensed health care person for a therapeutic purpose deemed necessary by that licensed healthcare professional.
The use of UC-MSCs, exosomes, and any related biological products are NOT approved by the FDA for the intended purpose to heal or cure a disease or condition. For FDA approved drugs or surgeries intended to medically help you please consult your physician for options.
Platelet-Rich Plasma: Platelet-Rich Plasma, PRP for short, is a regenerative procedure for joint pain, nerve pain, and can be used aesthetically. The procedure involves drawing typically 10-30n milliliters of the patients blood (About 1-2 tablespoons). Anticoagulant is added to the blood and it is spun in a centrifuge at a specific speed and time. During this process the red blood cells are separated from the the plasma portion of the whole blood and the platelets are concentrated. These concentrated platelets are then used for the treatment due to their innate healing abilities. Platelets provide the essential growth factors FGF, PDGF, TGF-ß, EGF, VEGF, and IGF that are involved in stem cell migration, differentiation and proliferation. The plasma is essential for cell survival as it contains nutrients, vitamins, hormones, electrolytes and proteins. Proteins are key molecules for the coagulation process and the formation of the fibrin polymer that will serve as a scaffold for cell migration, differentiation and proliferation.
While PRP can have a dozen or more growth factors the umbilical cord tissue/WJ can contain over 80 growth factors as well as an extensive amount of cytokines, mRNA, exosomes, secretomes and additional biologic elements including stem cells.
What’s your stem cell process?
I’ve heard of bone marrow and adipose stem cell treatments. What is the difference between using stem cells from umbilical cord tissue and the other treatments?
Another downside to these other treatments is the invasiveness. Both are procedures where the bone marrow or fat has to be removed from your body in surgical procedures, processed, and then injected back into you. This can be more painful, timely, and costly. The side effects of bruising and pain of these procedures can last for days to weeks.
What conditions can be treated with regenerative medicine therapies?
The conditions that can be treated with regenerative medicine is growing rapidly. The physician determines what conditions can safely be treated, and what kind or how much regenerative medicine would be clinically best for each patient. As with all other areas of medicine, we do not guarantee or claim that regenerative medicine/stem cell therapy will cure any disease. Our specific treatment protocols have NOT been evaluated by the FDA. Our treatments our guided by years of research and clinical experience to determine what will provide the best outcomes. Here’s some of the most common successful conditions we’ve treated:
- All types of arthritis (Osteoarthritis has had great success)
- Soft Tissue Conditions: Tendonitis, Bursitis, Ligament Injury (e.g. Achilles, Knee, Rotator Cuff)
- Joint pain (Low Back pain/Knee/Shoulder/Elbow/Hand/Wrist/Ankle/Neck/)
- Low Back Pain: Bulging or herniated discs, sciatica
- Autoimmune conditions: Multiple Sclerosis, IBD (Crohn’s disease/Ulcerative Colitis), Lupus, Rheumatoid arthritis
- Autism
- Cardiovascular health/Heart Failure
- Chronic Kidney Disease
- COPD/Respiratory disorders
- Diabetes
- Peripheral neuropathy
- Plantar fasciitis
- Seizures
- Sexual dysfunction for men and women (Erectile dysfunction, low arousal, orgasm disorder)
What are Mesenchymal Stem Cells?
Mesenchymal Stem Cells (MSCs) are a type of stem cell that can self-renew by dividing and can differentiate and change into a wide range of specialized cells with specific functions, including bone, cartilage, adipose tissue, connective tissue, and muscle.
These stem cells are found throughout tissue in our bodies. Due to their unique properties, MSCs have the potential to heal damaged bone, cartilage, tendon, tissue and reduce inflammation by modulating the immune system, changing into different types of tissue cells, and by signaling the surrounding cells to heal and grow. Over the past decades MSCs, particularly those derived from donated umbilical cord tissue, have made huge improvements in how we treat certain conditions in medicine.
How do Mesenchymal Stem Cells work?
How do umbilical cord mesenchymal stem cells heal the body?
Umbilical cord mesenchymal stem cells (UC-MSCs) (AKA Wharton’s Jelly) are a type of adult stem cell that can be isolated from the umbilical cord tissue and have been studied for their potential therapeutic use in tissue repair and regeneration. The exact mechanisms by which UC-MSCs promote tissue healing are not fully understood, but it is thought that they may work through a combination of different mechanisms, including:
- Differentiation: UC-MSCs can differentiate into various types of cells, such as osteoblasts (bone cells), chondrocytes (cartilage cells), and adipocytes (fat cells), which can help to repair and regenerate damaged or lost tissue.
- Paracrine signaling: UC-MSCs can release various growth factors and other signaling molecules that can promote tissue repair and regeneration. These signaling molecules can also modulate the immune response, which can help to reduce inflammation and promote healing.
- Immunomodulation: UC-MSCs can also modulate the immune response directly, which can help to reduce inflammation and promote healing.
- Tissue repair: UC-MSCs can also be used for direct tissue repair by transplanting them into the damaged area, where they can differentiate into the required cell types and promote tissue repair
How are the UC-MSCs administered?
There are several ways to administer umbilical cord mesenchymal stem cells to the body for treatment purposes. At Innate Healthcare Institute, we administer stem cells depending on each patient’s unique requirements and physical condition.
Intravenous (IV)
Using IVs is a safe and simple method for delivering stem cells throughout the body when done correctly. There is no need to use anesthesia, and it takes about 20-30 minutes to administer stem cells to the body through IVs. The cells must be properly prepared and filtered. Before the cells are administered, we will often deliver a specialized formula IV to help prepare the body for the cells (aka as priming) and help deliver the cells past the lungs (unless the goal is to have the cells stay in the lungs). Our IVs are tailored to each individuals needs.
Intramuscular (IM)
This method to administer stem cells involves directly injecting the stem cells into muscle. Intramuscular implantation is also a safe method that does not require anesthesia unless absolutely necessary. We will often combine IV and IM administration to synergistically improve the treatment as research shows cells delivered IM stay in the body longer and cells delivered IV tend to work faster.
Intranasal
An intranasal procedure involves transplanting the cells/exosomes/medicines into a specific area in the back of the sinuses, called the sphenopalatine ganglion. A specialized catheter (SphenoCath for Adults) or device (M.A.D. – Mucosal Atomization Device for smaller children) is inserted into the nose to deliver the medicine to a specific area by trained personnel. This method allows for frequent and targeted treatments to the brain for neurological and head, eyes, ears, and nose conditions.
Direct Injection
Stem cells are also administered directly to the hairline via injection or to the face for aesthetics. It can also be applied topically to achieve the same goal, depending on the condition and requirement for the patient.
Intraperitoneal
An intraperitoneal injection is a type of injection that is administered directly into the peritoneal cavity, which is the space within the abdominal cavity that contains the organs of the digestive system.
This type of injection is usually done using a small needle inserted through the abdominal wall and into the peritoneal cavity under the guidance of an ultrasound machine. The injection is then given into this space, allowing the medicine to be absorbed into the bloodstream through the peritoneum, a thin layer of tissue that lines the cavity. This approach is best used for any digestive or gastrointestinal conditions.
Are donated UC-MSCs safe to use?
The body’s immune system does not consider umbilical cord-derived mesenchymal stem cells (UC-MSCs) as a foreign threat. It means that there is no chance for your body to reject the stem cells we administer during the treatment. We have successfully administered stem cells for treating a wide range of health conditions without any instances of rejection.
The high-quality UC-MSCs we use at Innate Healthcare Institute for stem cell therapy in Phoenix, AZ, are also more efficient at differentiating and self-renewal than older stem cells that already exist in your body.
UC-MSCs have proved safe for a variety of conditions. For instance, in the 2017 study titled Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure. The authors concluded:
“Intravenous infusion of UC-MSC was safe in this group of patients with stable heart failure and reduced ejection fraction under optimal medical treatment. Improvements in left ventricular function, functional status, and quality of life were observed in patients treated with UC-MSCs.”
When used properly by a trained professional the risk of adverse events, infections, or complications are significantly minimal. This is why it’s so important to use a dedicated and experienced clinic like Innate Healthcare Institute.
I heard using stem cells in the United States was illegal. How are you able to use them for treatment purposes?
In general, the FDA has no jurisdiction over physicians and clinics. The 10th Amendment of the U.S. Constitution regulates the governance of healthcare to the states, the federal government has almost no authority over how physicians treat patients, including stem cells.
Where the rumor, “The FDA says it’s illegal to do stem cells” comes from is from clinics promoting “cures”, “treatments” etc using stem cells, and/or not even using stem cell medicine at all. So, it’s more about the bad players that harming patients or false advertising. Both the FDA and FTC have authority to punish medical practices for making public advertising claims about unsafe, untested, or unapproved procedures. However, they still have no authority to take action against a physician for counseling a patient within the confines of a consultation. The key issue in this situation is whether the patient fully understands the treatment, the risks involved, and has given proper consent. If this is the case, and the treatment has at least reasonable scientific validity for the physician to recommend the treatment, the FDA still has no authority to take action against the physician.
What is illegal is when medical professionals make claims their treatments can cure a disease, or something along those lines. Or when a medical professional states they’re using stem cells for a therapeutic purpose when they are not. This gets clinics and doctors into trouble as they listen to commercial labs that sell biological “stem cell” products without any actual stem cells in them.
Off-Label Use: It is a bit of a grey area as to how much enforcement authority the FDA has over a physician using even a stem cell “drug” in his own private practice. However, the FDA still has no official authority over a physician practicing in a particular manner he/she deems safe and effective in his/her independent medical judgment. While the FDA can regulate stem cells if they are considered drugs, it cannot regulate a physician using the drug in a responsible way that is unapproved by the FDA.
Another reason the umbrella phrase “Stem cells are illegal…” is thrown around a lot is because of the distribution of stem cell products. If we were to culture stem cells and sell these cells to doctors, clinics, hospitals, etc, then we would need to apply for a Biologics License Application and we would essentially be manufacturing a drug. We DO NOT sell stem cells and are not a commercial lab.
We’ve gone the extra mile to provide umbilical cord derived mesenchymal stem cells for the use of experimental therapies. We’ve spent hundreds of thousands of dollars on the process of developing live umbilical cord mesenchymal stem cells for the purpose of experimental treatments.
State Law: Arizona permits naturopathic physicians to diagnose and treat disease with a wide range of procedures and natural medicines. In the state of Arizona, it is legal for us to administer stem cells. This can be a very confusing topic.
So, while this may be an issue for some doctors in other states, the state regulatory board in Arizona allows us to administer stem cells. However, we can’t and don’t make any claims that stem cells can treat or cure any condition. The use of any stem cell medicine is purely experimental.
The Use of Stem Cells in Medical Procedures in the US
A huge case was just decided in August of 2022 that ruled in favor of a doctor here in US administering stem cells, from adipose in this instance, for medical procedures. Essentially the FDA took a doctor/medical practice to court claiming they were using stem cells as a drug. I’ve often gotten a lot of negative comments in these FB groups when I try to tell others that you can get expanded umbilical cord MSC procedures in the US, it just greatly depends on where you go. Hopefully this will educate those that are unaware or deter those that are spreading myths (or lies).
USA (FDA) vs California Stem Cell Treatment Center
Findings of Fact and Conclusions of Law
You can google the case and maybe find the PDF on the injunction. The long and short of the outcome was that if a physician is taking material that’s in the human body, removing it and putting it back into the human body, that’s not a drug. This isn’t my sole interpretation of the case, I discussed this with my FDA healthcare attorney (who had already known about the case).
FDA’s claim is that if you’re altering it in anyway then it’s a drug. The Court is saying that’s not the case, altering stem cells from the human body isn’t the same as manufacturing a drug. Even if you’re changing the cells it still doesn’t constitute using the cells as a drug. Therefore, anyone using stem cells for a medical procedure is outside the regulations of the FDA.
Only thing is if you’re trying to sell stem cells (or biologics in general) across state lines is when it gets messy. Or if you’re changing the cell from one type from another. Therefore, taking stem cells from an umbilical cord, expanding them, and using them for a medical procedure is a legal practice if the state medical board allows it.
A letter from our lawyers
What makes Innate Healthcare Institute’s stem cell therapy unique?
Innate Healthcare Institute is currently the only clinic in the U.S. to use live, expanded UC-MSCs in dosages only seen in clinics over-seas. It’s important to expand the cells to get an adequate number of cells for effective dosages in treatments. We are a private clinic and produce this form of experimental medicine for our patients only. A licensed physician at Innate Healthcare Institute can determine if UC-MSC therapy is for you.
We are also unique in our holistic and integrative approach to medicine. We believe there is a place for the old and the new, the ancient and the modern ways of healing can be combined for a more comprehensive approach. Not everyone aligns with this view, and we’re ok with patients only wanting stem cell treatments. It’s your body, time, and money. Whenever possible we do like to utilize laboratory testing, herbal medicines as well as pharmaceuticals, nutrition, lifestyle changes, mind-body medicine, Chinese medicine, and Naturopathic Medicine, in addition to modern use of regenerative medicine.
A common complaint we hear from patients that have ventured to clinics overseas is that they don’t hear from the staff after they leave the clinic. Unless they want to pay for another treatment, there’s little to no follow-ups. Our success only depends on the success of our patients. So we have a responsibility to make sure you’re getting the best healthcare possible.
Being located in Phoenix, AZ makes us very accessible and easy to communicate with. Telehealth visits are simple and if further treatments are needed it’s easy for us to ship medications to our patients. Traveling to our clinic is much simpler then traveling overseas.
In addition, our prices are often a fraction of the price of most overseas clinics. We believe very strongly that Americans shouldn’t have to travel abroad to have access to this amazing medicine. There have been clinics that have sprung up over the years offering very low prices on “stem cell” treatments, however you should be very careful and make sure you know the full extent of what you’re getting. The phrase “You get what you pay for” can definitely apply here.
How does payment work?
As of October 5th, 2023, our payment protocols have been updated. We require a 50% deposit to schedule the appointment and start preparing for the first treatment. The remaining 50% will be due prior to receiving treatment when you arrive at the clinic on the first day of the treatment series. For patients who would like to explore financing options, we recommend using Advanced Care Card services at www.advancedcarecard.com
The website will prompt you to “Apply Now” and redirect you to their processing website. There, you will be asked a series of questions regarding current financial status and offered a loan agreement with the dollar amount approved and the interest (if any) applied to the loan. If the patient is satisfied with the terms, they will receive a credit card in the mail 7-14 business days after the loan was accepted that can be used towards medical expenses.
How are Innate Healthcare Institute (IHI) treatments different from clinics in Mexico or other overseas?
A few of the biggest differences we’ve noticed is providing higher doses of cells, more comprehensive treatments and lab testing, better follow-ups and continued care, better affordability, and better delivery of stem cell treatments. Cost is always a big concern so make sure you’re comparing ‘apples to apples’ when deciding between clinics.
Some clinics in Mexico have good reputations while others are very hit and miss. Some of the pros we hear about good clinics in Mexico is they offer high amounts of umbilical cord cells at a better price than many clinics in the US. However, a downside may be that you don’t know how well the cells are sourced or prepared and what are the doses. Another complaint is many patients don’t like the lack of feedback they get from going to clinics in Mexico. They only get the treatment of stem cells, but no other care or follow-ups. If it doesn’t work or something goes wrong from the treatment, there’s no accountability from the clinic. At IHI we encourage follow-ups, track patient data for improvements and research, and if somethings not working, we take a lot of pride and responsibility in our patients to get them feeling their best.
We’ve also been privy to outright poor ethical practices from clinics overseas. We’ve been told reps from clinics in Mexico have told potential patients dozens of lies about our clinic to persuade them to go to their clinic. We would never stoop so low to do this and are very big advocates of cooperation over competition. One owner of a very popular clinic in Mexico contacted us to buy “fresh” stem cells from them and have them shipped to our clinic here in Phoenix. He raved and raved about how much better his “fresh stem cells” are compared to cryofrozen cells. When asked how it was possible to ship UC-MSC and still have them be “fresh” he ignored the question, and many others asked, and continued to guarantee his cells are the best. A ‘fresh’ stem cell transplant can only be done on-site where the cells are being cultured, and it’s a very difficult, timely procedure and has a higer risk of contamination. If you ship them, you must freeze them a special way or the cells will die. It’s impossible to do what he claimed. This is very unethical practice and came from a clinic we previously thought was very reputable.
Some clinics in Mexico have very well-trained doctors and staff, however some have very minimally trained personnel. We once had a patient tell us about her failed treatment from a clinic in Mexico. She went for her severe and chronic back pain and got a single injection. This raised flags immediately cause a comprehensive back treatment can have at least 5 different injection sites, minimum. She later found out the doctor that did the treatment was an OB-GYN. So it’s important to find out who’s doing the treatments and what their experience is. While it may sound enticing to get have a single, minimalist treatment and save a few bucks, you may regret it if it doesn’t work at all or something goes wrong from an untrained person administering the treatment.
Another ‘perk’ many clinics in Mexico (and many clinics overseas) add is bundling rides to and from the airport and hotel stays into the treatment plans. This is certainly appealing and takes a lot of stress of the patients, so if that resonates with you go for it. However, one needs to remember that you’re still paying for all that in the cost of the treatment. Hotels and fancy rides aren’t magically free. We prefer to let our patients stay where they prefer and focus on the treatments. If you want to save money and stay in an Airbnb or live it up at one of Phoenix’s many resorts that’s up to you.
There are other concerns to worry about when traveling to foreign countries. Safety is always a concern and unfortunately many areas are very unsafe for Americans to travel to. As one patient put it, “I’d rather travel here to Phoenix and not have to worry about renting an armored car to get treatments there”. Another patient told us a story of her friend that went to a clinic in Mexico for a Brazilian butt lift procedure. After returning she fell very ill and went to the ER. The doctors remove d the wrappings from her surgery and found out she had a kidney removed during her cosmetic surgery procedure! It was more of a joke but the notion that traveling abroad can be very dangerous. Another safety concern is infections from food and drinks. This can be minimalized but is still a concern Americans should be aware of.
Many overseas clinics are vastly more expensive than IHI. We’ve heard of clinics in countries such as Panama, Cabo, or the Cayman Islands that charge $20,000 + for a single treatment. The cell numbers they administer are minimal as well, not even over 100 million cells in most cases. While these clinics can have some obvious success, it’s mostly due to the fact that stem cell medicine can be amazing. We feel it’s poor practice to charge such high rates when multiple treatments produce the best outcomes. It’s nearly impossible for most people to get continued care at those rates, not to mention the excessive travel.
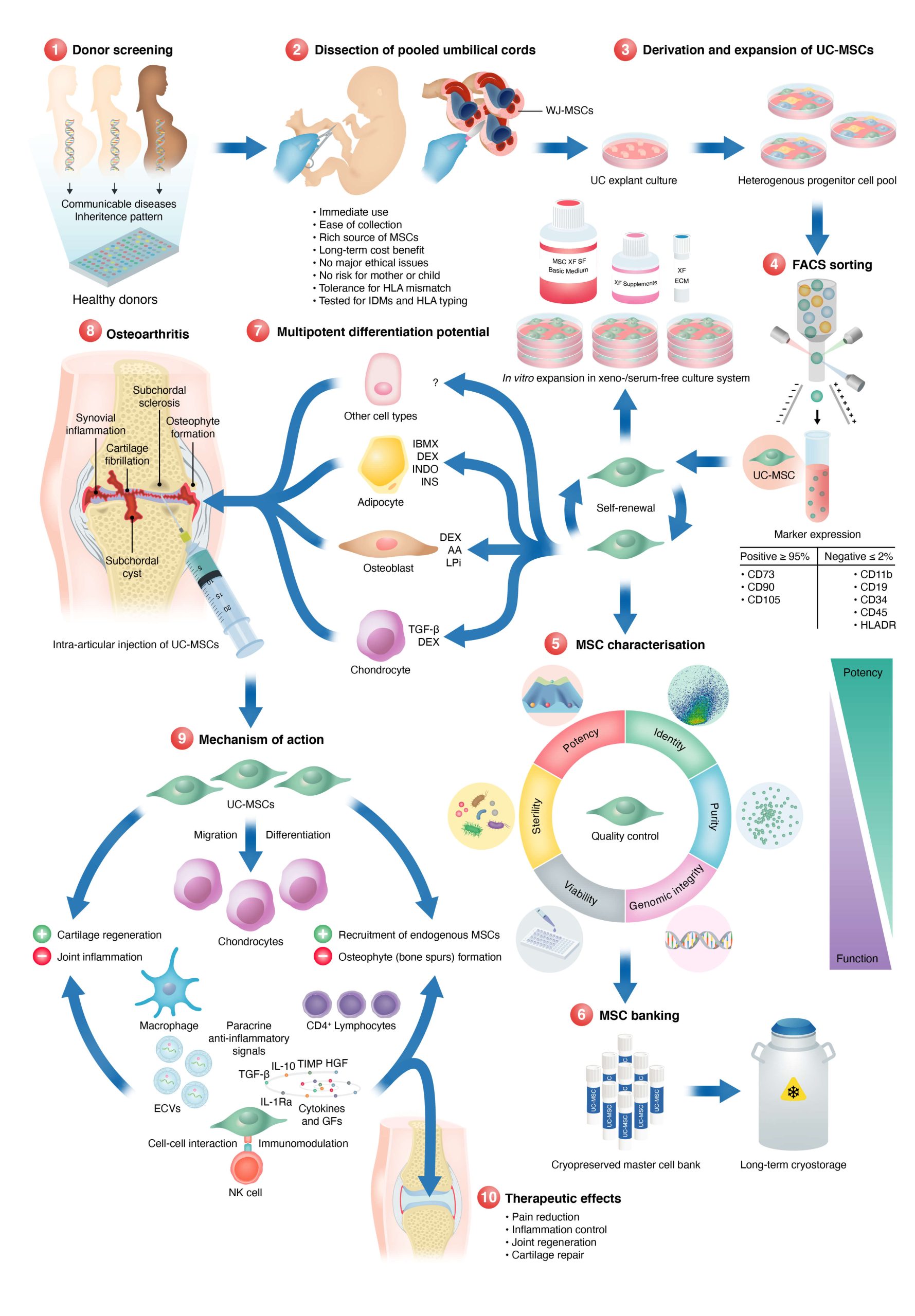
How are umbilical cord stem cells cultivated/ cultured and in what media?
The umbilical cords are processed by a non-enzymatic procedure. Stem cells are isolated from the Wharton’s Jelly of the umbilical cord. Pure populations are isolated by using markers cd 73, 90 and 105. They are cultured in 10 layered stacks supplied by Corning (adherent dishes) which have 0.22 uM filter caps. Each vial is cryopreserved in cGMP grade DMSO free cryo medium. Every cell batch is checked for mesenchymal stem cell markers (CD 73, 90 and 105), fibroblast morphology, cell proliferation, population doubling, cell communication and differentiation into multiple cell lineages.
We also sequence micro RNAs that are secreted by our UC-MSCs (exosomes) required for paracrine signaling.
We grow our cells in the best medium with defined growth factors and rich supplements ideal for maximum cell growth and potential to differentiate to other cell types, repair, regenerate and regulate/reset an impaired immune system. Work has been done on these topics for over 30 years and we have the best cells for treating major diseases as of today.
Do you use fresh or frozen cells?
Cryopreservation of cells is a very standardized practice, however the myth that “frozen cells are dead” persists. This myth mainly refers to the fact that commercial biological products are frozen and don’t have any live cells in them. But they never had any live cells to begin with. Many representatives in Facebook and other social media groups will claim fresh cells are the only way to get a treatment, and any frozen cells used in the US are dead. These are blatant lies to persuade people to go to clinics they represent that are claiming their “fresh” stem cells are the best. It’s purely marketing. Cryopreservation of cells has been around for a long time. Research has shown there’s no major differences between fresh and cryopreserved cells. Fresh stem cells certainly sound ideal, and they are an amazing treatment (if you’re really getting fresh cells) but don’t be fooled by sneaky marketing tactics.
We’ve been contacted by clinics in Mexico offering to sell us their “fresh” stem cells. When we ask “how are they fresh if you’re going to deliver them to us?”, they dance around the question and only talk about how great their cells are. So, we think this new wave of clinics in Mexico promising fresh cells might be a marketing gimmick. Remember any clinic that promises you “fresh” cells is trying to convince you to come to their clinic, all of us stem cell clinics are banking on our cells being the best. So of course, they’re going to sell you on that so you can travel all the way to Mexico instead of staying here in the states.
Just make sure you’re doing your due diligence when considering a clinic offering both
Where do we get our stem cells?
Have the donors of the umbilical cords donated been given the COVID vaccine?
Do you perform karyotyping?
Will putting stem cells from an umbilical cord increase my chances of getting a genetic disease?
No. We go back 2-3 pedigree generations and make sure the cord donors don’t have any genetic diseases. It’s not possible to test for diseases unless they have the genetic disease.
If the cells are normal, the best way to make sure they are normal is to sequence the whole genome and look if there are any anomalies. This is one of the ways we know our cells are healthy and effective for healing.
Not every cord processed is used. Some reasons why a cord might not be used:
- Cells might grow slowly
- They don’t pass the differentiation test
- Morphology is different
- Population doubling time is not normal
- Variable CD markers
- Cell migration assay is no good
We test all of the above 6 assays and make sure the cells perform the best before selecting them for medical treatments.
How long do you culture the stem cells? Are they safe?
P0 takes 1 month, P1 takes 15 days from P0, P2 takes 15 days from P1. We never stress our cells to grow more than 1-2 passages. So these cells have all the original potency to repair, regenerate, respond to cytokines and secrete paracrine factors and exosomes with the necessary dosage once inside the body.
The cells are received from donated banks that are carefully screened for infectious diseases. We retest the cells from certified labs.
These cells are not cancerous since:
- The morphology seen under the microscope is normal for mesenchymal stem cells. Cancer cells have varied morphologies.
- Cancer cells divide very fast (4-6 hrs per division) compared to 22-24 hrs per division for umbilical cord mesenchymal stem cells.
- We constantly monitor (2-3 times a day) these cells for normal cell division, growth kinetics, markers on their surface and their differentiation potential to neurons, heart cells, cartilage, skeletal muscle cells (to name a few).
What do exosomes do in our body?
How long do exosomes stay in our body?
Will the DNA from the stem cells mix with mine?
It will not mix with our DNA, but it will be a part of the body.
If we eat meat and plants, their DNA will be a part of our body too! The human DNA is a mix of bacterial, viral, fungal , protozoan and human DNA. We are only 70% human by genome.
There should be no issues of integration of MSCs DNA into our DNA. Only viruses have that capability.
The MSCs injected will never be transferred onto the next generation.
Side effects of using high doses of umbilical cord stem cells?
Clinical trials have been done using 6-10 million cells per kg body weight.
I have seen some unique side effects in 3 (to date) menopausal women with IV administration. They get “hot flashes” type of symptoms while administering. Which resolve within minutes.
How many exosomes are produced from stem cells?
How are exosomes derived from umbilical cord stem cells?
Why are the cells not kept in a rubberized bottle?
What type of conditions can UC-MSC therapy treat?
UC-MSC therapy can treat a growing number of conditions with increasing research into the possible applications for the revolutionary treatment.
The physician determines what conditions can safely be treated and what kind or how much regenerative medicine would be clinically best for each patient. As with all other areas of medicine, we do not guarantee or claim that regenerative medicine/stem cell therapy will cure any disease.
Please note that the FDA has NOT evaluated our specific treatment protocols. The FDA still considers umbilical cord mesenchymal stem cell therapy as experimental but has recognized its safety over the years. Our treatments are guided by years of research and clinical experience to determine what will provide the best outcome. Here are some of the most common conditions we’ve treated successfully at Innate Healthcare Institute over the years:
- All types of arthritis (Osteoarthritis has had great success)
- Soft Tissue Conditions: Tendonitis, Bursitis, Ligament Injury (e.g., Achilles, Knee, Rotator Cuff)
- Joint pain (Low Back pain, Knee, Shoulder, Elbow, Hand, Wrist, Ankle, and Neck)
- Low Back Pain: Bulging or herniated discs, sciatica, OA, degenerative disc disease, facet and sacroiliac joint dysfunction, damaged muscles, tendons, and ligaments.
- Autoimmune conditions: Multiple Sclerosis, IBD (Crohn’s disease/Ulcerative Colitis), Lupus, Rheumatoid arthritis
- Autism
- Cardiovascular health/Heart Failure
- Chronic Kidney Disease
- COPD/Respiratory disorders
- Diabetes
- Peripheral neuropathy
- Plantar fasciitis
- Seizures
- Sexual dysfunction for men and women (Erectile dysfunction, low arousal, orgasm disorder)
* If you have a condition not on the above list, contact us to see if we’ve treated it recently.
How do stem cells work to improve longevity and age-related symptoms?
Umbilical cord mesenchymal stem cells (UC-MSCs) have been proposed as a potential therapeutic option for a wide range of diseases and conditions, including age-related diseases, but their safety and efficacy in this area are still being evaluated.
There is some evidence from preclinical studies that UC-MSCs may have anti-aging effects by promoting tissue repair and regeneration, modulating the immune response and reducing inflammation.
One of the hallmark signs of aging is a loss of our intrinsic MSCs. UC-MSCs may serve to replenish our MSCs and promote direct repair at sites of tissue injury throughout the body. There are several mechanisms on which it is thought UC-MSCs can promote longevity and anti-aging:
- Tissue repair and regeneration: Stem cells, such as UC-MSCs, can differentiate into various types of cells, such as osteoblasts (bone cells), chondrocytes (cartilage cells), and adipocytes (fat cells), which can help to repair and regenerate damaged or lost tissue.
- Paracrine signaling: Stem cells can release various growth factors and other signaling molecules that can promote tissue repair and regeneration. These signaling molecules can also modulate the immune response, which can help to reduce inflammation and promote healing.
- Immunomodulation: Stem cells can also modulate the immune response directly, which can help to reduce inflammation and promote healing.
- Telomere maintenance: Some studies have shown that stem cells may help to maintain telomere length, which are the protective repetitive sequences that are found on the end of chromosomes. They shorten as we age and the shortening of telomeres is associated with aging and age-related diseases.
- Prevention of oxidative stress: Some studies have shown that stem cells may have antioxidant properties that can help to prevent oxidative stress. Oxidative stress is a major cause of aging, and it can lead to the damage of cells and tissues.
- Mitochondrial Transfer: improves mitochondrial functions and cellular performance, including protein translation of respiratory complexes, ROS overexpression, mitochondrial membrane potential, mitochondrial morphology and bioenergetics, cell proliferation, mitochondrion-dependent viability, and apoptotic resistance.
A 2017 study in mice (Cell Death and Disease (2017) 8, e2996; doi:10.1038/cddis.2017.316) revealed that UC-MSC have the capacity to regulate the aging brain and may be a potential intervention for combating a decline in cognition in aging. Another study in mice found that transferring young MSCs significantly slows the loss of bone density and prolongs the life of old mice. (SCIENTIFIC REPORTS | 1 : 67 | DOI: 10.1038/srep00067 )
Another study that looked at frailty found that an ideal dose of 100 million UC-MSC was not only safe and well tolerated, but showed improvements in functional an immunological status. Individuals showed increased in their 6 minute walk test and decreases in inflammation markers (TNF alpha). It’s important that more studies be done in this field. (Journals of Gerontology: MEDICAL SCIENCES, 2017, Vol. 72, No. 11)
It is also important to note that the aging process is complex and multifactorial, and it is not clear if UC-MSCs alone would be sufficient to improve the effects of aging. But they could play a significant role when combined with other anti-aging therapies.
What is the best stem cell source?
How many stem cells do I need?
Your Innate Healthcare Institute physician will help guide you through recommended doses that we use most frequently and successfully. If it’s a condition we haven’t treated or haven’t treated often we will review research to see the best dosages.
How can I find a reputable provider for UC-MSC treatments?
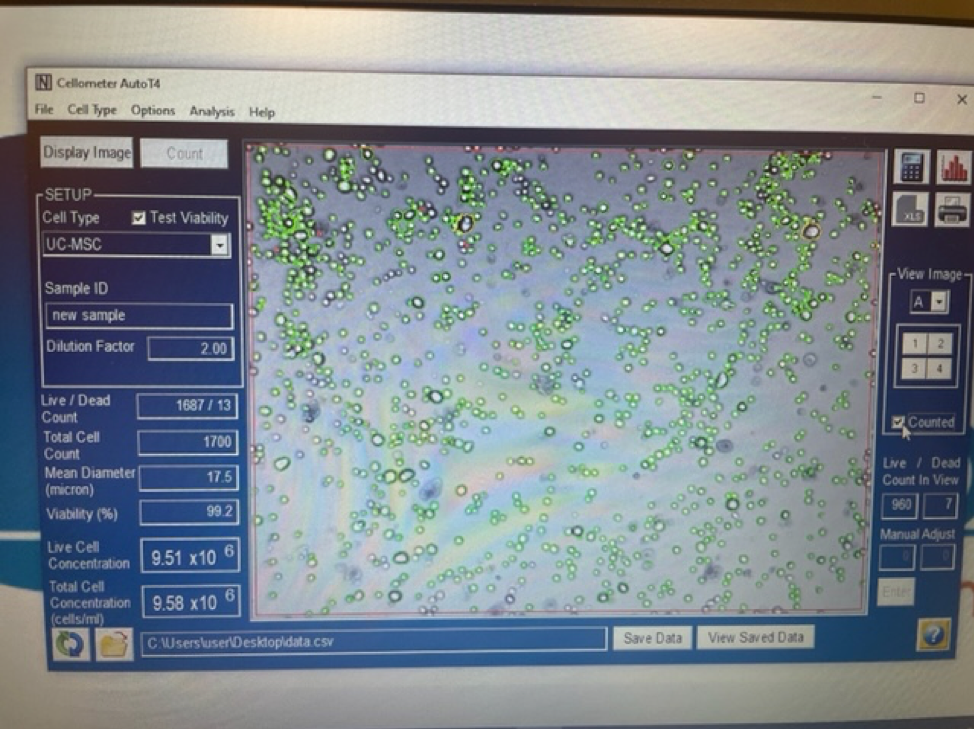
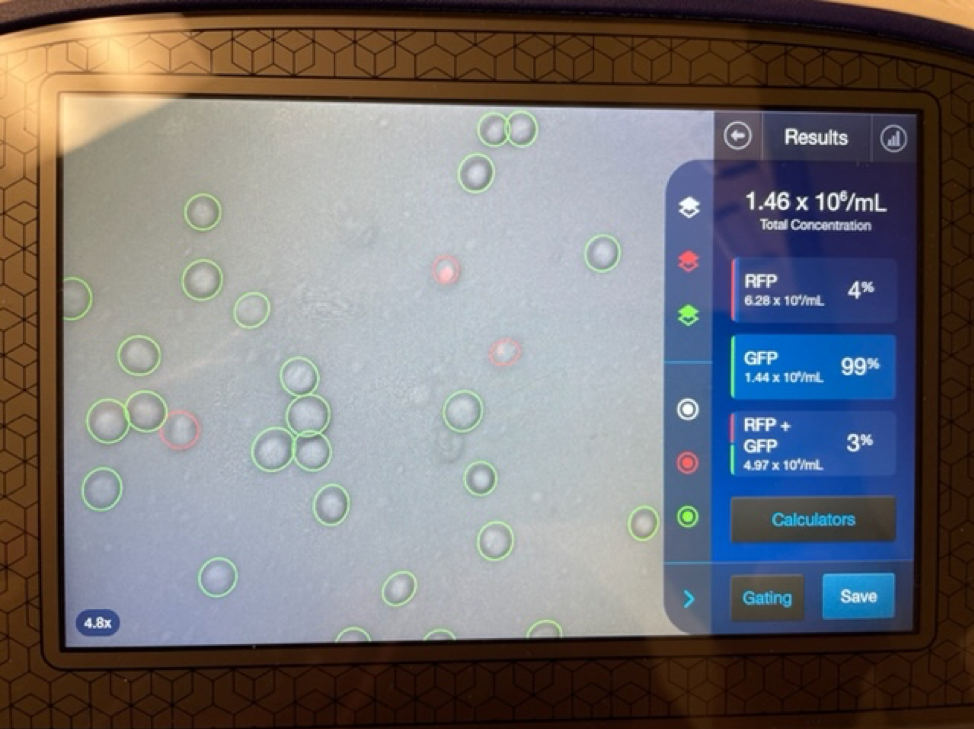
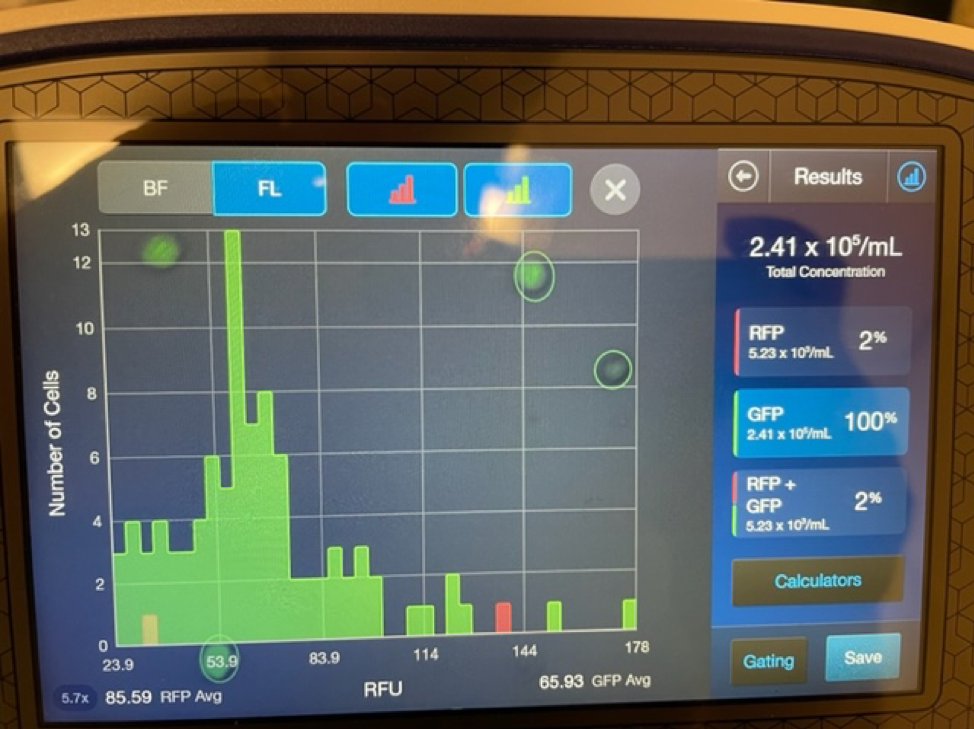
It is important to first find out if you are getting a true stem cell treatment or not. Due to the rise in popularity and success of stem cell treatments many clinics and marketing agencies have freely used the term stem cell therapy. Clinics that falsely promote stem cell treatments will typically tell potential patients that they are treating with “5cc’s of stem cells” or “you will receive 6 units of stem cells”. These are not accurate ways to quantify a stem cell treatment. Umbilical cord mesenchymal stem cells are measured in the number of cells delivered. 1 million stem cells can be added to 5CC’s of saline just as easy as 100 million stem cells.
An experienced UC-MSC clinic can be verified by showing cell viability and flow cytometer testing like the ones we’ve provided below:
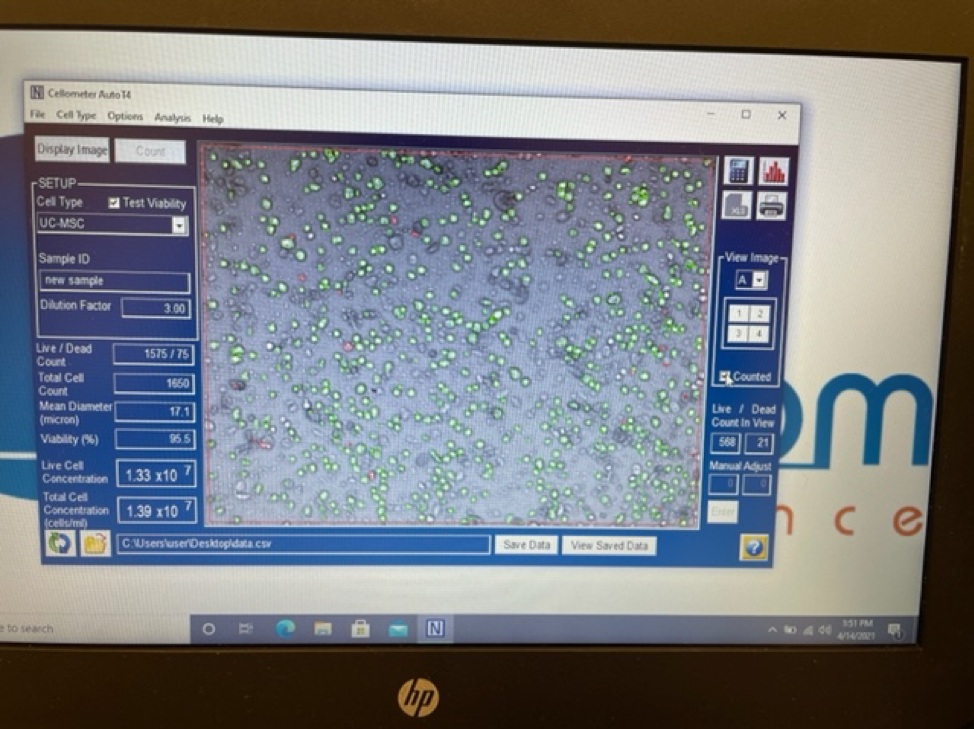
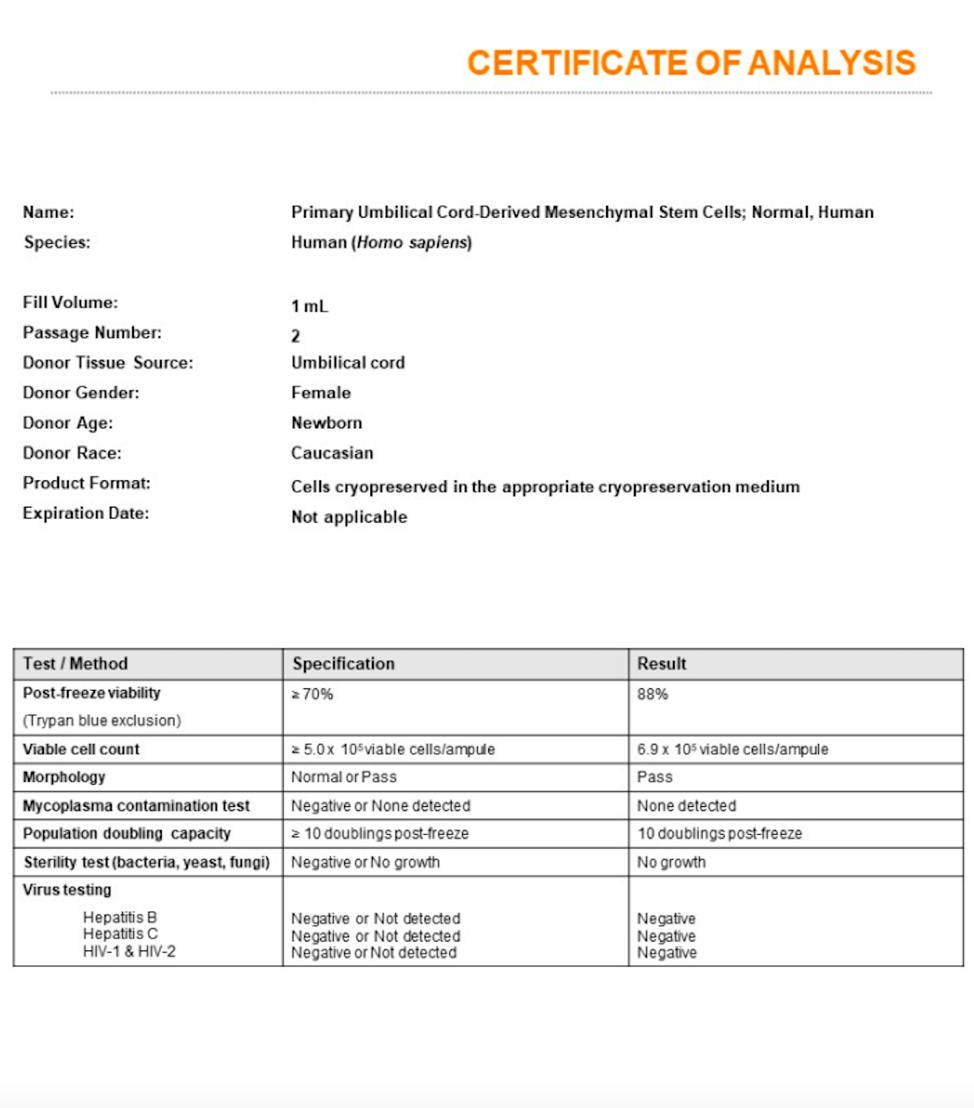
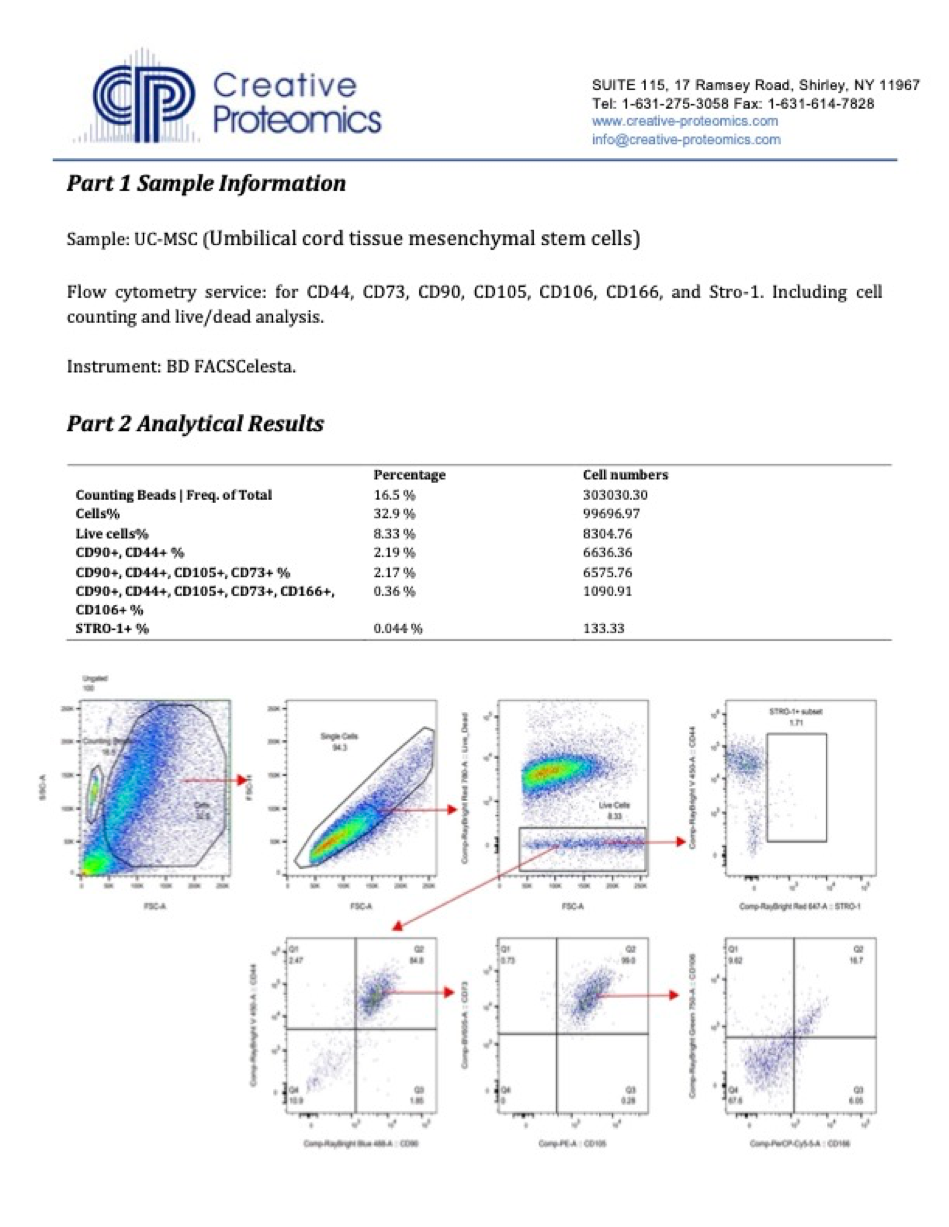
Are UC-MSC treatments for covered by insurance?
How fast can I expect to see results after UC-MSC therapy?
This is why at Innate Healthcare Institute we implement a comprehensive and holistic treatment plan to expedite the healing process as much as possible. Are dosages of cells and effective administration methods has shown that we get faster and longer lasting results than many other clinics we’ve compared to from talking to other patients.
What are the doctors’ credentials at IHI?
The doctors at IHI are mainly comprised of Naturopathic Medical Doctors (NMD) and Medical Doctors (MD). However other healthcare professionals may also make up the comprehensive team at IHI, including, Chinese Medicine Doctors, acupuncturists, massage or physical therapists, energy healers, medical assistants, imaging specialists, medical aestheticians, etc.
A naturopathic medical doctor is a licensed healthcare practitioner who has completed a four-year postgraduate program at a naturopathic medical school. Naturopathic medical schools offer comprehensive training in both conventional and alternative medicine, with a focus on the use of natural therapies to promote healing and prevent disease. Naturopathic doctors are educated and trained in accredited naturopathic medical colleges, which are approved by the U.S. Department of Education. They diagnose, prevent, and treat acute and chronic illness to restore and establish optimal health by supporting the person’s inherent self-healing process.
Naturopathic medical doctors (NDs) combine their knowledge of modern medical science with a range of natural healing modalities, including herbal medicine, clinical nutrition, acupuncture, homeopathy, and lifestyle counseling. They work to identify and address the underlying causes of illness, rather than just treating the symptoms. Naturopathic medical doctors also emphasize the importance of disease prevention, patient education, and individualized care.
In the United States, naturopathic medical doctors are licensed in some states, and they are recognized as primary care providers in many of these states. While many naturopathic doctors are trained in primary care, like conventional medical doctors (MDs), some choose to specialize or focus their practices. They work in a variety of healthcare settings, including private practices, clinics, hospitals, and research centers.
A medical doctor, also known as a physician, is a licensed healthcare professional who has completed medical school and received a Doctor of Medicine (M.D.) or Doctor of Osteopathic Medicine (D.O.) degree.
Medical doctors are trained in the diagnosis, treatment, and prevention of illnesses and injuries, and are responsible for maintaining the overall health of their patients. They can specialize in various fields such as internal medicine, pediatrics, surgery, cardiology, neurology, and many others.
Medical doctors typically work in hospitals, clinics, private practices, and other healthcare settings, and they may also be involved in research, education, and administration. They examine patients, order and interpret diagnostic tests, prescribe medications, perform medical procedures, and provide counseling and education to promote wellness and disease prevention.
Will the UC-MSC be rejected by my body?
Umbilical cord tissue-derived mesenchymal stem cells (UC-MSCs) have been found to be relatively immunoprivileged, which means they have the ability to evade or suppress the immune response of the recipient’s body. This property of UC-MSCs is thought to be due to several factors, including:
- Low expression of major histocompatibility complex (MHC) antigens: UC-MSCs have low expression of MHC class I and II antigens, which are proteins on the surface of cells that help the immune system recognize foreign cells. This low expression of MHC antigens makes it more difficult for the recipient’s immune system to identify and attack the UC-MSCs as foreign cells.
- Production of immunosuppressive factors: UC-MSCs can secrete various immunosuppressive factors, such as indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and transforming growth factor-beta (TGF-beta), which can help to suppress the immune response and prevent rejection of the cells.
- Modulation of immune cells: UC-MSCs can interact with various immune cells, such as T cells, B cells, and natural killer (NK) cells, and can inhibit their activation or induce a regulatory phenotype, which can help to reduce inflammation and prevent rejection of the cells.
We’d love to hear from you.
Don’t see your question? Click the button below to contact us. If you prefer immediate assistance, please call us at 602-603-3118.
